A Multi-Disciplinary Center Developing New Tools and Therapeutics
Oncology
Targeted Delivery of Drugs to Cancer Cells: Changing the Face of Chemotherapy
Professor of Biomedical Sciences Maurizio Pellecchia of the UCR School of Medicine, in collaboration with the Cedars-Sinai Medical Center, is developing innovative strategies to target specifically cancer cells for the selective delivery of chemotherapy. These strategies are based on the EphA2 receptor that is expressed mainly on the surface of cancer cells where it promotes cell proliferation, tumor angiogenesis, cancer cell migration and metastases. The collaborative groups are investigating the approach against prostate cancer, melanoma, and pancreatic cancers.
Read more about targeted drug delivery to cancer cells
Targeting Apoptosis via Chemical Design
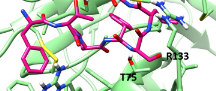
In tumor cells, deregulation of programmed cell death, also known as apoptosis, provide cancer cells a growth advantage, and confers resistance to chemo- and immuno-therapy and radiation. Two classes of proteins, namely anti-apoptotic Bcl-2 proteins, and IAPs, are responsible for defective apoptosis in cancer cells. Professor of Biomedical Sciences Maurizio Pellecchia of the UCR School of Medicine is developing novel agents targeting several members of these two classes of proteins using innovative approaches. In collaboration with researchers at UC San Diego, these agents are being sought to revert resistance to chemotherapy in Chronic Lymphocytic Leukemia (CLL), while a collaborative study with the City of Hope aims at developing the agents against Acute Myelogenous Leukemia. The agents are also effective in disease models of several solid tumors such as prostate, lung, and breast cancers and potentially other tumors, and collaborative studies are ongoing with the Cedars-Sinai Medical Center in these tumors.
Read more about Targeting Apoptosis
Targeting a Specific Metastasis-Promoting Form of the Prolactin Receptor in Breast, Ovarian and Prostate Cancer
Professor Ameae Walker and colleagues have designed novel splice modulating oligonucleotides (SMOs) that decrease expression of the proliferation-promoting and metastasis-promoting form of the prolactin receptor without affecting expression of other forms of the prolactin receptor. This extreme specificity is the next step in highly targeted therapeutics designed to reduce the possibility of untoward side effects. Approximately 95% of breast, ovarian and prostate cancers express prolactin receptors and all cancer stem cells examined so far are positive for the prolactin receptor. This compares favorably with the 70% of breast cancers that express estrogen receptors and suggests utility of the SMOs in triple negative breast cancers, chemotherapy resistant ovarian cancer and castration resistant prostate cancer. Published work to date has shown that the administered SMOs were extremely effective at reducing lung and liver metastases in two highly aggressive metastatic models of breast cancer: BT474 human xenografts, used for testing Herceptin, and a 4T1 syngeneic mouse model, which allowed testing in an animal with an intact immune system. An international patent has been filed and has completed one round of review.
Read more about targeting specific prolactin receptors
The SUMO and Ubiquitin Pathway as Targets for Anti-Cancer Therapeutics
The lab of Professor Jefferson Perry uses macromolecular crystallography and in-solution small angle x-ray scattering technologies to study the molecular mechanisms of signal transduction pathways controlling genome stability. In particular, the lab is studying the roles of components of the SUMO and ubiquitin pathways, in addition to kinase accessory proteins, which have critical roles in human health and disease. Current research projects include translational aspects, where the Perry laboratory uses fragment-based ligand discovery and computer-aided drug discovery methods as part of the rational-based drug discovery process. The lab is focusing on various targets that likely cause resistance to therapy in breast cancer, acute myeloid leukemia, chronic myeloid leukemia or multiple myeloma, as part of collaborations with labs at UCR, UC San Diego, The Scripps Research Institute and the City of Hope.
Read more about research into anti-cancer therapeutics
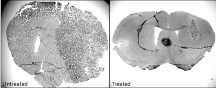
Novel Strategies to Combat Glioblastoma Multiforme (GBM)
The lab of Associate Professor Emma Wilson collaborates extensively with lecturer Jack Eichler of the chemistry department to teach undergraduates about glioma and develop novel drug therapies from design and synthesis through to in vitro and in vivo testing. Glioblastoma multiforme is a devastating cancer that even with all available therapies gives a life expectancy of ~ 18 months. This collaboration has discovered several novel metal based compounds and associated ligands that have significant anti-glioma efficacy against murine and human glioma cell lines and one in particular that demonstrates significant in vivo efficacy. Further work is being conducted to understand the mechanism of action of these compounds.
Read more about research into combating Glioblastoma multiforme
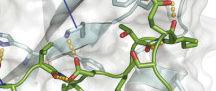
Selective Proteasome Inhibitors Against Multiple Myeloma and Solid Tumors
The laboratory of Distinguished Professor Michael Pirrung in the UCR Department of Chemistry has developed a family of natural product-based proteasome inhibitors, the syrbactins. Proteasome inhibitors are approved drugs for treating multiple myeloma. In collaboration with structural biophysics groups, the interactions of these compounds with one of the seven unique catalytic sites of human proteasomes have been elucidated. One syrbactin, TIR-199, has passed several hurdles in the National Cancer Institute’s Developmental Therapeutics Program. It is about to enter tumor xenograft and other preclinical studies, and its commercialization is being pursued via a spin-off company, Hibiscus Bioscience, founded by Pirrung and his collaborator, Professor Andre S. Bachmann, Ph.D. of Michigan State University's College of Human Medicine. Among proteasome inhibitors the syrbactins are unique in that they are cyclic and have a defined conformation, which affects their selectivity for proteasome catalytic subunits. Using this property, compounds have been discovered that target catalytic sites otherwise difficult to address, such as the trypsin-like subunit of the immunoproteasome.
Read more about selective proteasome inhibitors against multiple myeloma and solid tumors
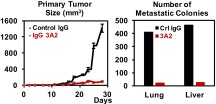
Highly Selective Monoclonal Antibody Treatments for Metastatic Spread of Breast Cancer
For more than 20 years, scientists have been developing drugs that block a family of matrix metalloproteiases (MMPs) which play important roles in tumor progress and metastasis. However, clinical trials on a variety of promising small molecules all failed because these inhibitors lack the specificity needed to target faculty MMPs while still allowing "good" MMPs to perform their regular cellular duties. Inspired by convex shaped paratope of camelid antibodies, Assistant Professor Xin Ge and colleagues of the Bourns College of Engineering generated a panel of highly selective monoclonal antibody inhibitors of MMP-14 with nanomolar potency. In highly metastatic, syngeneic, orthotopic model of breast cancer, IgG 3A2 markedly inhibited growth of the primary tumor, but more significantly reduced metastatic spread to the lungs and livers by 94%. This project is supported by NIH and in collaboration with Professor Ameae Walker of the Division of Biomedical Sciences.
Cancer Genomics
Professor Adam Godzik’s group is developing computer algorithms to analyze cancer genetic alterations in the context of the three dimensional and network structures of proteins they are affecting. Understanding which structural features of cancer proteins are affected by alterations such as mutations and splicing variants can lead to discovery of novel cancer driver events and genetic correlates of drug responses and cancer outcomes and provide hypotheses about how they can be potentially remediated. Both the results and the tools to identify novel insights from such integrated genomic/3D structural analysis are available on the cancer3D server, developed and maintained by the Godzik’s group.
Target Score for Therapeutic Antibodies
Therapeutic antibodies are quickly becoming leading drugs in our fight against cancer and autoimmune diseases. Many of the steps of developing such antibodies are streamlined, but identifying targets of intervention remains a manual process. Professor Adam Godzik's group has developed an Al approach to integrate expression data from diseased tissues with available clinical and biochemical information to prioritize targets for therapeutical antibodies. the target score for Therapeutic Antibodies Targets (TABT) identifies known targets in the top 5% of the highest scoring candidates and suggest hundreds of possible new targets for further analysis.
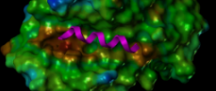
Cyclic peptides as inhibitors against oncoproteins with disordered regions
The lab of Professor Min Xue focuses on developing peptide-based drugs against challenging oncology targets such as transcription factors. The Xue group leverages unique cyclic peptide-based libraries to access 3D diversifiable chemical space, which promises better affinity and specificity against disordered protein epitopes. With the support from NIH and DoD, the group has developed several lead compounds targeting MYC and MMP2. The group is actively collaborating with multiple institutes, including UCLA, ISB, and Harvard, to test those peptide leads' biological and therapeutic implications.
READ MORE ABOUT CYCLIC PEPTIDES AS INHIBITORS AGAINST ONCOPROTEINS WITH DISORDERED REGIONS